Bond Order Of N2
So N-N bond length should be shortest in N_2 because of its triple bonding but N_2 Bond is more stronger than any other Nitrogen Compound. A single bond has a bond order of 1 a double bond has a bond order of 2 and a triple bond has a bond order of 3.

Calculate The Bond Order Relative Stability Of The Following 1 N2 2 O2 3 O2 4 O2 Superoxide 5 Chemistry Chemical Bonding And Molecular Structure 10568655 Meritnation Com

Solved B2 Use The Molecular Orbital Diagram For N2 Below Chegg Com
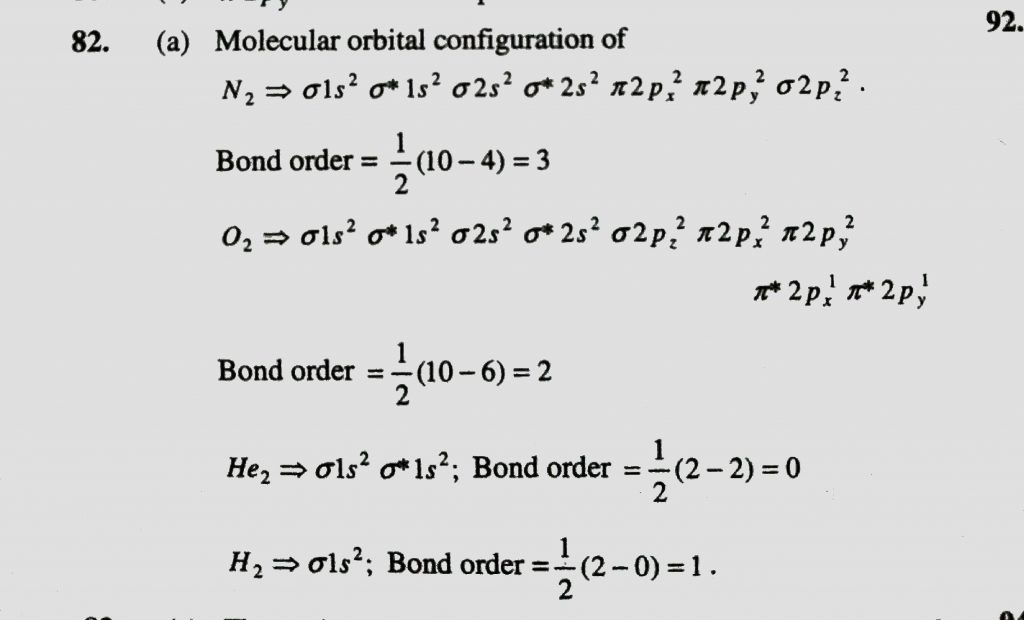
Which Of The Following Has The Highest Bond Order A N2 B O2 C He2 D H2 Sahay Lms
In advanced inorganic courses you will learn about group theory which is a tool to predict the energies of different molecular orbitals.

Bond order of n2. It has a formula of Bond order Number of electrons in bonding - Number of electrons in. The bond order of N2 is 3. A Number of electrons in bonding molecular orbitals.
Na N a is the. Bond Order N2 Formula. Bond order of N2 3.
It is defined among the number of bonding and antibonding electrons. The bond order of N2 is 3. In the Lewis structure of the N2 molecule there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
If there are unpaired electrons present in the orbitals then it is paramagnetic otherwise diamagnetic. For the Bond Order of N2- is 25 which is Nitrogen ion. The correct order of increasing N - N bond stability of N22- N2 N2 N2s is a N22- N2 N2s N2.
Both nitrogen gas N2 and hydrogen gas H2 are diatomic molecules. Also more antibonding electrons lead to. Bond order of N2121041263 so it has 3 bonds.
No special bond order formula is usually required. Bond order refers to the general strength or energy of a bond. VSEPR model assumes that molecular geometry minimizes the.
Click to see full answer. For the Bond Order of N2 - is 25 which is Nitrogen ion. What is the bond order of N2.
N2 has less bond energy. Bond order could be calculated by the following formula. By using a combination of group theory.
The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. Electronic configuration of N 2 14 electrons KKσ2s 2 σ2s 2 π2Px 2 π2py 2 σ2pz 2. If there are unpaired electrons present in the orbitals then it is paramagnetic otherwise diamagnetic.
Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. Bond order NbNa 2 B o n d o r d e r N b N a 2. Nb Number of electrons in bonding moleclar orbitals.
Bond order of both N2 and N2- is 25 but according the molecular orbital theory N2- has more antibonding electrons than N2. N2 N2 d N22- N2 N2s N2. Bond order 12 nb-Na where.
Electronic configuration of N2 σ2s2 σ2s2 n2px2 n2py2 n2pz2. They both have non-polar covalent bonds. Write the molecular orbital diagram of N2 and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric.
Bond order for N2 is 3. Bond Order 1 2nb na 1 2 n b n a. Explain What is the relationship between bond order and.
Bond order of N 2 1 2 10 4 1 2 6 3 so it has 3 bonds. Energy diagram of N 2 is. Which is Nitrogen molecule.
What is the bond order of N2. Determining bond strength for N2 N2 N2- N2. Bond order of N2 N2- and N2 will be.
The bond order of N2 is 3. Bond order is calculated by the following formula. Number of bonds during a molecule is additionally referred to as bond order.
For the Bond Order of N2 - is 25 which is Nitrogen ion. But first we look at the diagram of molecular orbitals for N2 the bond order for the nitrogen molecule is 3. This is because according to molecular orbital theory it has fewer electrons in bonding orbitals.
Express the bond order numerically. Nb N b is the number of bonding orbital. Energy diagram of N2 is.
Write the electron configuration of a N2 molecule. The upper the bond order the stronger the pull between the 2 atoms and also the shorter the bond length. So it is the longest Bond Order in all Nitrogen Compounds.
It has triple bonding. Bond order for N2- is and bond order for N2 is I have not included pictures of the MO diagrams that show the orbital energies. Bond order 12 10 4 Bond order 3.
Bond Order Number of bonding electrons Number of antibonding electrons 2. Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. The bond order of N2 nitrogen nitrogen molecule has a triple bond so the bond order is three.
The higher the bond order the more energy needed to break the bond. For the purposes of this class N2 and N2- will be considered equal as they both have a bond order of 25. B Number of electrons in antibonding molecular orbitals.
Is N2 paramagnetic or diamagnetic. Bond order of N_2 is 3. I Structure of N 2.
The MO method for N2 gives the bond order equal to 25. Lets remember that the. Which is Nitrogen molecule.
Bond order formula is given as below latexBond order frac12left a b right latex where.

N2 Contains A Triple Bond What Molecular Orbitals In N2 Contribute To The Triple Bond Do You Think Each Of The Molecular Orbitals Involved In The Triple Bond Contributes Equally To The

Calculate The Bond Order Of N2 Chemistry Questions
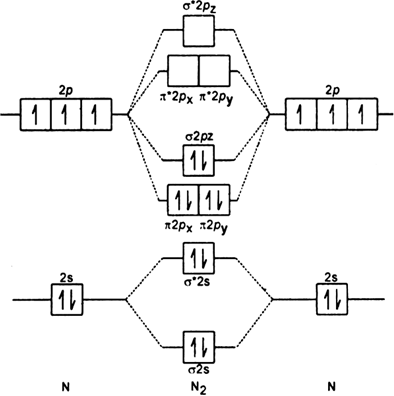
Discuss The Formation Of N2 Molecule On The Basis Of Mo Theory Predict Its I Bond Order Ii Magnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium

What Is The Bond Order Of N2 Brainly In
The Correct Order Of Increasing N N Bond Stability Of N2 2 N2 N2 N2 S Is Sarthaks Econnect Largest Online Education Community
What Is Meant By The Term Bond Order Calculate The Bond Order Of N2 O2 Sarthaks Econnect Largest Online Education Community
What Is The Bond Order Of N2 Quora
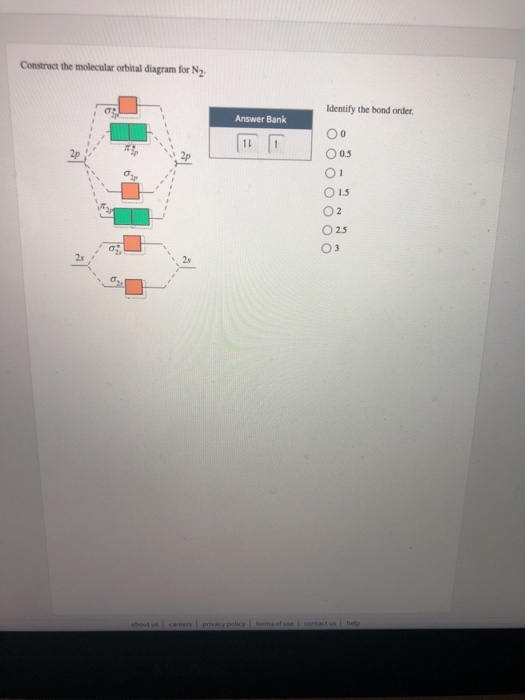
Solved Construct The Molecular Orbital Diagram For N2 Chegg Com
0 Response to "Bond Order Of N2"
Post a Comment