Molar Mass Of Sulphur
Hence molecular mass of Fe Atomic mass of Fe 56 g. The RMM is used in many sorts of calculations in chemistry and so you must be able to calculate it to answer all the other calculations you might meet.
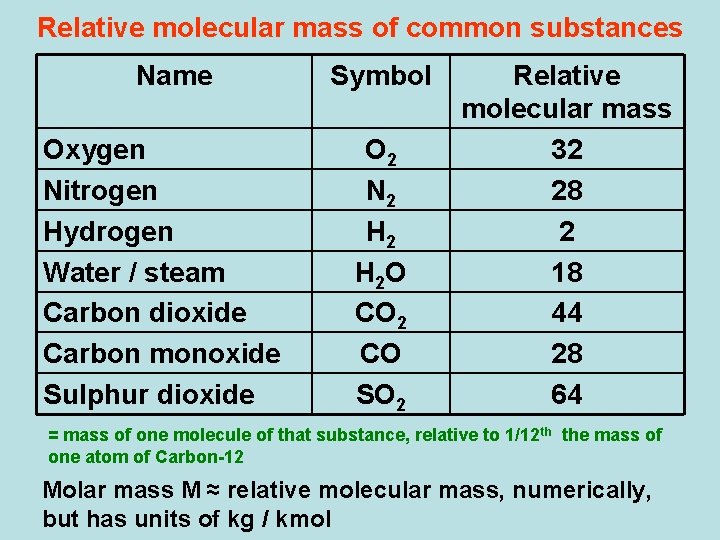
Chemistry Lower Secondary Ydp Whiteboard Exercise The Molar Mass Of Sulphur Dioxide

Calculate The Number Of Molecules Of Sulphur S8 Present In 16 Gram Of Solid Atomic Mass Of Sulphur Brainly In

Sulphur Molecule Exists As S8 How Many Moles Of Sulphur Are Present In 25 6 Grams Of Sulphur
Chemical elements listed by melting point.
Molar mass of sulphur. The characteristic mass m o is defined as the analyte mass that gives an integrated absorbance of 00044 s. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Sulfur in nontechnical British English.
Sulphur 0625 moles. Lenntech European Head Office Distributieweg 3 2645 EG Delfgauw The Netherlands Phone. For an ideal gas the internal energy - u - is a function of temperature.
Answer 1 of 11. Fe is a monoatomic molecule. Sulfur is the tenth most common element by mass in the universe.
How many atoms in 3 moles of sulfur. The unit eq a keq is 1000 eq refers to molar equivalent of potential acidity resulting from eg. So the reaction is taking place as follows.
Element Symbol Atomic Molar Number mass g mol1 The value given in parenthesis is the molar mass of the isotope of largest known half-life. Core Practical 1Measure the molar volume of a gas Mass of CaCO3 in g Volume of CO 2 in cm 3 Analysis From the graph read the volume of CO2 given off with 025 g CaCO3 Work out the moles of CaCO3 in 025g 0251001 25 x 10-3 Assume the moles of CO2 moles of CaCO3 Work out molar volume of CO2 volume of CO2 moles of CO2. Extract 020 cm3 of propanone into a hypodermic syringe and then measure the mass of this syringe 2.
Du change in internal energy kJkg. Sulphur oxidised and reduced nitrogen as well as base cations. SO2g H2Ol -- H2SO3aq.
How many moles of carbon are in 522 x 1026 C atoms. Anand in Advances in Eco-Fuels for a Sustainable Environment 2019 13254 Calorific value. DT change in temperature K.
Using hand protection remove a gas syringe from the oven and note the volume of air already in the. Consider a 100 g of the compound. In other words the molar mass is the total mass of all the atoms in grams that make a mole of a particular molecule.
Mass 40 Given mass of Ca 79 mg 79. The molar mass of iron sulphur and oxygen is 55845 g mol 1 32065 g mol 1 and 15999 g mol 1. SF 6 has an octahedral geometry consisting of six fluorine atoms attached to a central sulfur atom.
The equation is balanced as written. Ratio of moles of copper and sulphur 0944 0625 3 2 15. They both dissolve in water and they both form fictitious acids.
Number of moles of Fe 01402. A Relative lowering of vapour pressure. I Electronic configuration.
Du c v dT 1 where. Specific heat c v varies with temperature but within moderate temperature changes the specific. The Bomb Calorimeter Model-IKA C2000 was used to measure the cross calorific value of the solid and liquid samples.
Cu S CuS. 1 as pressure increases the excess molar volume is positive and a maximum is observed at the region of highest isothermal. Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample.
Mass 56 Given mass of Fe 785 g. Definition and mathematical expression. What is the mass of 5 moles of sulphur.
Therefore number of moles of oxygen 64 g 32 g mol 1. It is often shortened to RMM. 79 mg of Ca at.
Sulfur hexafluoride SF 6 or sulphur hexafluoride British spelling is an extremely potent and persistent greenhouse gas that is primarily utilized as an electrical insulator and arc suppressant. Elements their Atomic Number and Molar Mass. By using the proposed method the obtained m o were similar among the evaluated sulphur-containing compounds and comparable to the literature data 42 which evaluated the same wavelengths and pixels as the ones used in this work and found m o equal to 75 ng.
Let me make it more clear with an example of sodium chloride. 407 x 10-5 moles 6. Oxygen in natural form will be molecular oxygen O2.
Example 2- Calculate number of moles present in 64 g of oxygen. The mass in g of 1 mole of a substance is known as the molar mass or molecular weight of the substance. Solution Molar mass of oxygen 32 g mol 1.
SO2 is much like CO2. C-13 is used for instance in organic chemistry research studies into molecular structures metabolism food labeling air pollution and climate change. It is inorganic colorless odorless non-flammable and non-toxic.
So it contains 279 g of iron 241 g of sulphur and 480 g of oxygen. 1 mole sulfur 602 x 1023 S. 255573 00001 58517 005 50356 020 59838 050 004 004 Some general trends can be identified in the excess molar volume plots.
31 152 610 900. Number of moles of Fe 785 g 56 g 01402. 2 grams of sulphur 2 32 0625 So 0629 mole of copper reacts with same mole of sulphur.
It is a constant-volume type calorimeter that measures the heat of a particular reaction or measures the calorific value of the fuels. Molar Mass Molecular Weight - The term mole also referred to as mol was first used by Ostwald in 1896. A change in internal energy can be expressed as.
It is abundant multivalent and nonmetallicUnder normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S 8Elemental sulfur is a bright yellow crystalline solid at room temperature. Equivalent and molar conductivityv ariations of conductivity with concentration. The Relative Molecular Mass of a compound is the sum of the masses of all the atoms present in the molecule.
Carbon isotopes and mainly C-13 is used extensively in many different applications. How many moles of sulfur are in 245 x 1019 S atoms. 785 g of Fe at.
If copper is increased to 6 grams. The moles of iron sulphur and oxygen are calculated as follows. Sulphur S 16 3206 Tantalum Ta 73 18095 Technetium Tc 43 9891 Tellurium Te 52 12760 Terbium Tb 65 15892 Thallium Tl 81 20437 Thorium Th 90 23204.
Elements by ionization energy. 6 gm of copper 6 635 0944 moles of copper. What is the mass of 8 moles of carbon.
Number of moles of Fe Given mass of Fe Molecular mass of Fe. The molar mass of the compound is unknown. The molar mass links the mass of a substance to its moles.
The molar mass of any substance can be calculated whose chemical formula is given. The molar gas volume 1m ol gas 24dm3 at room temperature and pressure Method for using a gas syringe to calculate the Mr of propanone 1. 1 keq N ha-1 yr-1 is equal to 14 kg N ha-1 yr-1 and 1 keq S ha-1 yr-1 is equal to 16 kg S ha-1 yr-1.
86682 moles or 867 x 102 moles Example 1. The molar mass of. Calculate the Molar Mass of sulphuric acid H2SO4 This molecule contains 2 atoms of hydrogen each of mass 1 2 x 1 2 g mol1 1 atom of sulphur of mass 32 1 x 32 32 g mol1 4 atoms of oxygen of mass 16 4 x 16 64 g mol1 1Total mass 98 g mol Example 2 Calculate the Molar Mass of lead nitrate PbNO32 Care.
Sulphur is a chemical element with the symbol S and atomic number 16. The molar mass of a compound defines the mass of 1 mole of that particular substance and the number of grams per mole of a compound. Therefore mass of sulphur in grams molar mass x number of moles 550 mol 320 g mol 1 1760 g sulphur.
Preparation properties and uses of sulphur-dioxide. And like the nonexistent H2CO3 there are no molecules of H. Thats the equation youre expecting.
C v specific heat for gas in a constant volume process kJkgK.
3

Energy Conversion Es 832 A Eric Savory Www
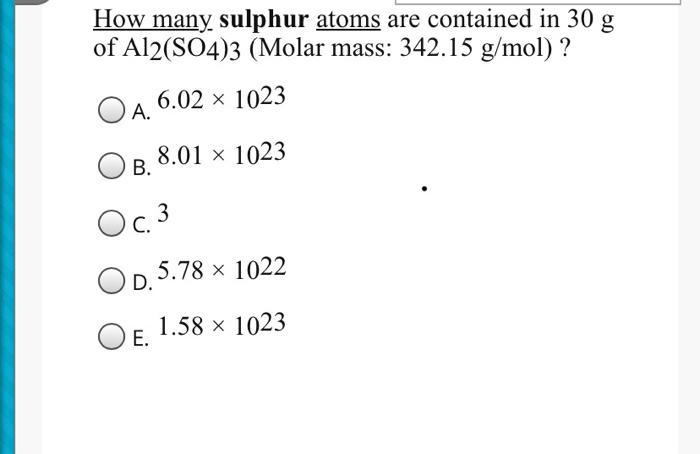
Solved How Many Sulphur Atoms Are Contained In 30 G Of Chegg Com
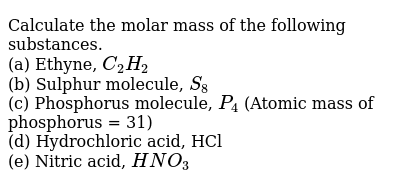
Calculate The Molar Mass Of The Following Substances A Ethyne C 2 H 2 B Sulphur Molecule S 8 C Phosphorus Molecule P 4 Atomic Mass Of Phosphorus 31 D Hydrochloric Acid Hcl E Nitric Acid
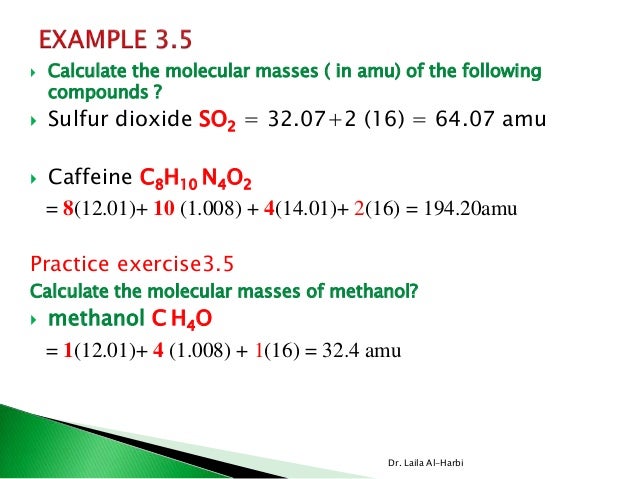
Chapter 3
What Is The Atomic Mass Of Sulphur Quora
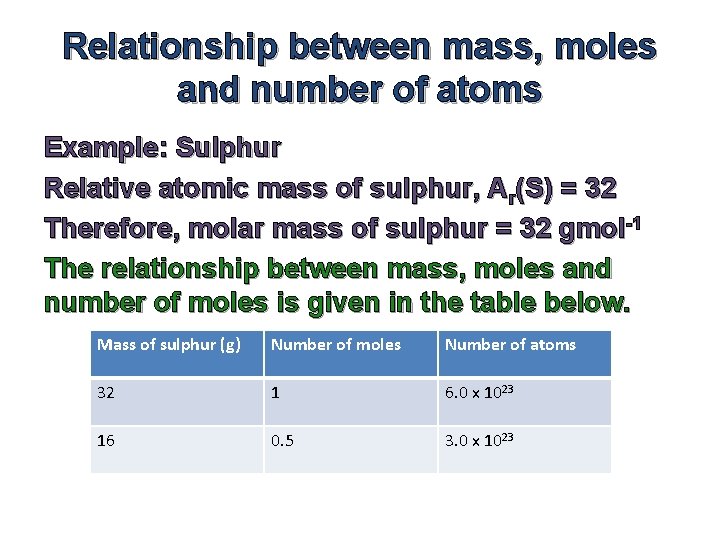
Chemical Formulae Composition Stoichiometry Definitions 1 Chemical Formulae
Atomic Mass Chemistry Socratic
0 Response to "Molar Mass Of Sulphur"
Post a Comment