Sodium Oxide Reacts With Water
Write Equations For The Following Reactions I Aluminium Oxide And Sodium Hydroxide Ii Zinc And Dilute Sulphuric Acid Iii Magnesium Nitride And Water I Chemistry Topperlearning Com Qd5fztyzz
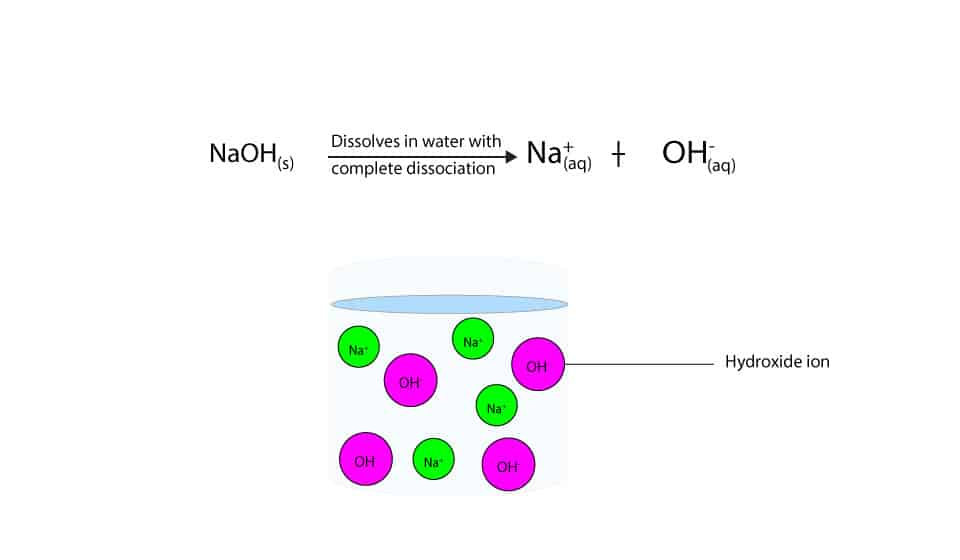
Why S Sodium Hydroxide A Strong Base While Ammonia A Weak Base

Solved Paragraph Styles 10 Sodium Oxide Reacts With Water Chegg Com

Sodium Hydroxide Naoh Is Classified As A Strong Base For Every Mole Of Sodium Hydroxide Added To A Large Volume Of Water One Mole Of What Ion Enters The Solution Socratic

Sodium Oxide Reacts With Water To Form Sodium Hydroxide Solution Which Is Course Hero

Give A Balanced Chemical Equation For The Reaction Of Water With Sodium Oxide

Equation For Naoh H2o Sodium Hydroxide Water Youtube

Word Equations Information Sheet
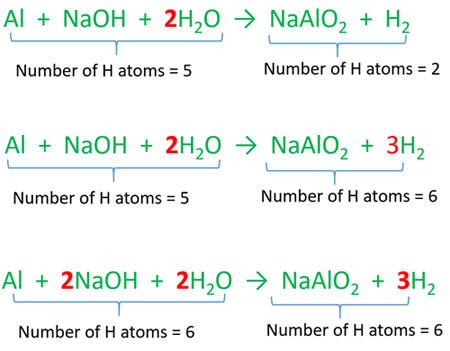
Aluminium And Sodium Hydroxide Reaction Al Naoh

4 1 Writing And Balancing Chemical Reactions Ppt Download
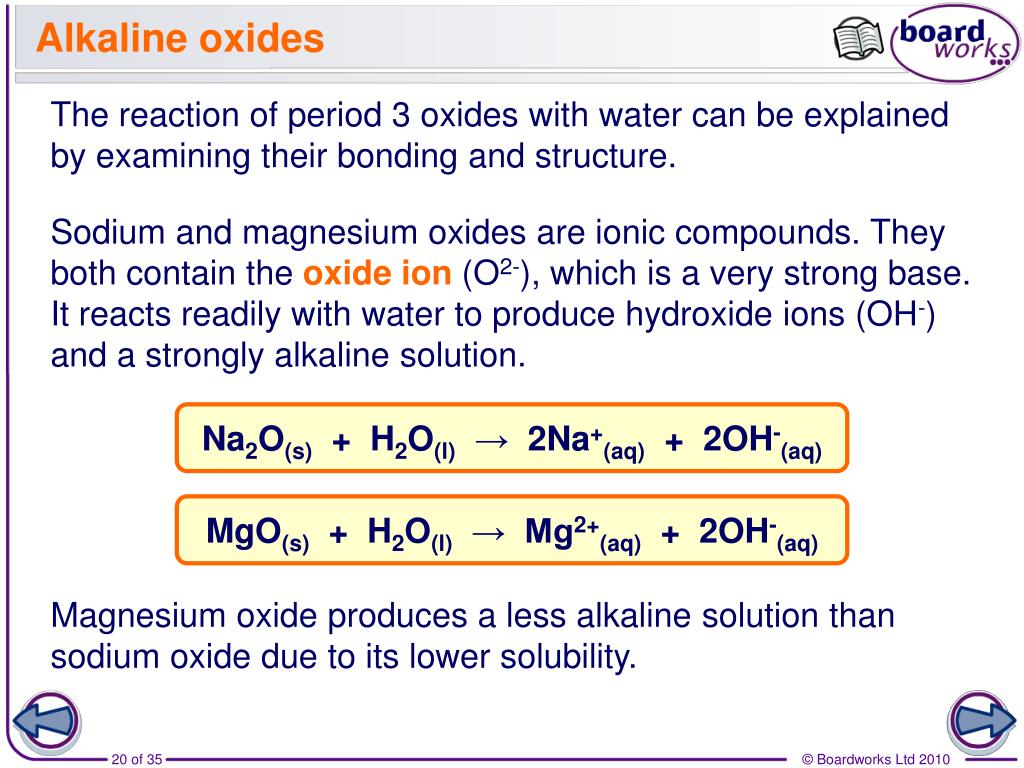
Ppt The Period 3 Elements Powerpoint Presentation Free Download Id 5604085
0 Response to "Sodium Oxide Reacts With Water"
Post a Comment